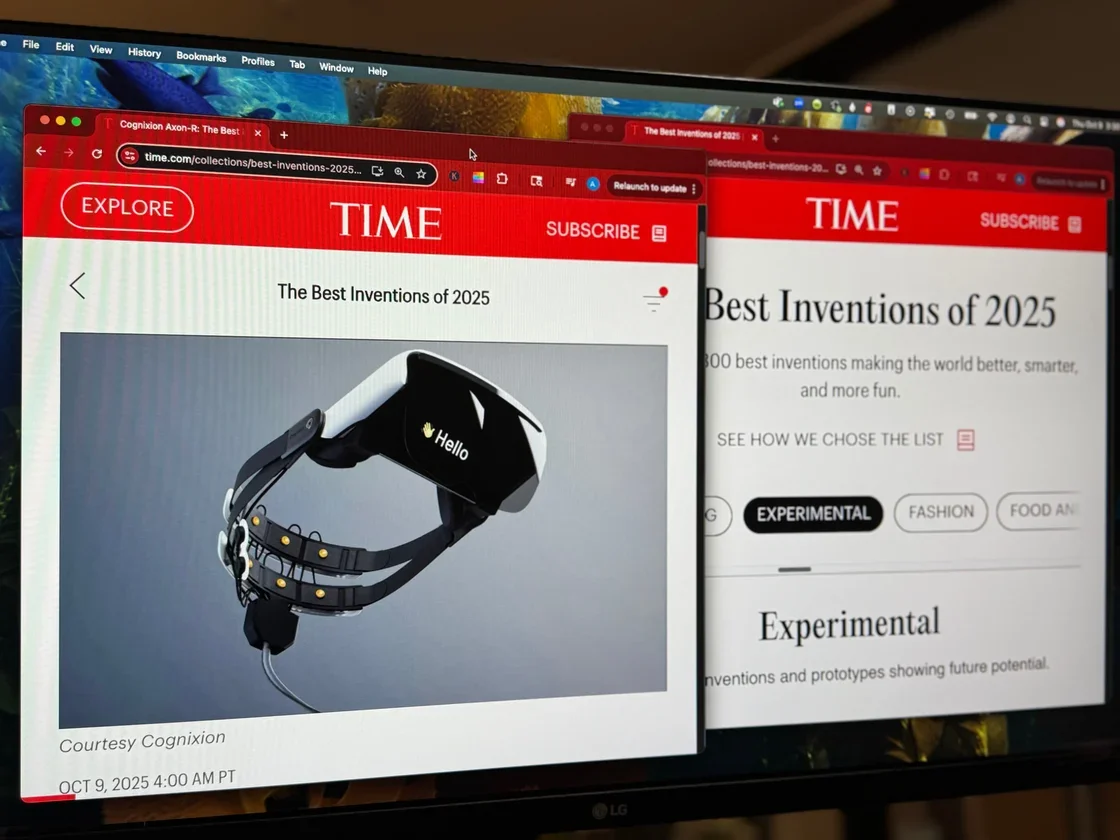
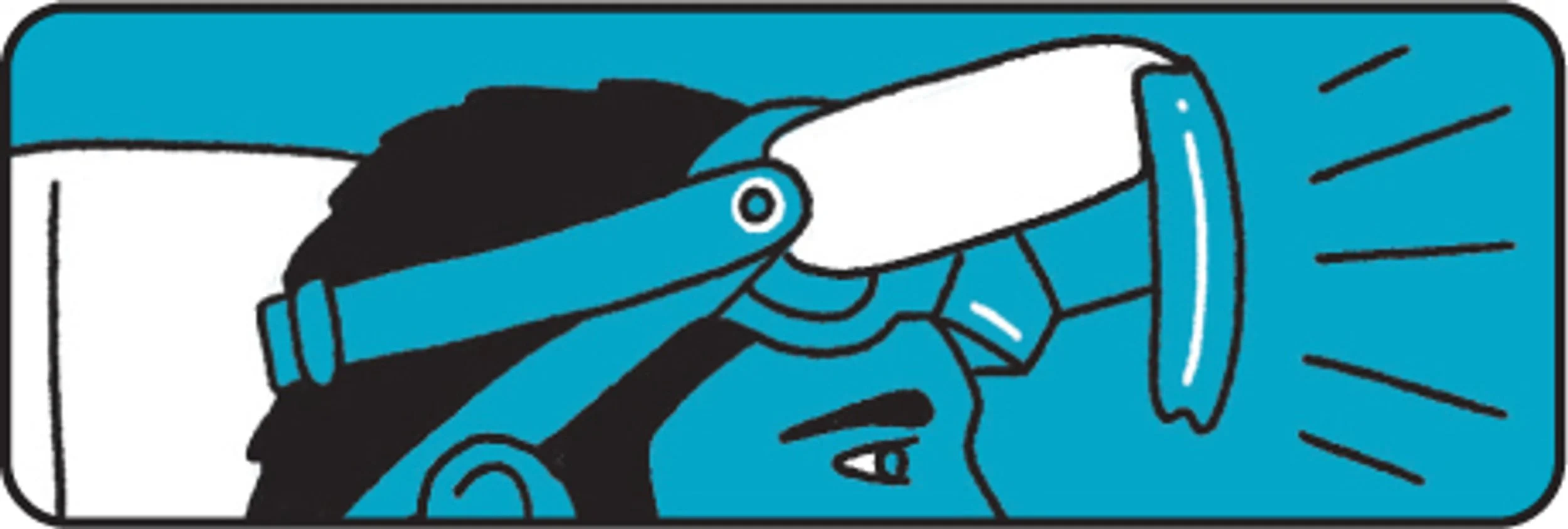
We are honored to share that Cognixion has been named a 2025 Tech for Global Good Laureate by The Tech Interactive. Our work will be featured in a special exhibition at The Tech Interactive for the next year.
Launching at The Tech Interactive’s eighth annual Tech for Global Good Celebration, taking place January 16 in San Jose. This year’s event also honors legendary technologist and philanthropist Steve Wozniak with the 2025 James C. Morgan Global Humanitarian Award, presented by Applied Materials, in recognition of his lifelong commitment to accessible, joyful learning and technology for positive change.
Unlock Your Mind: Neurotechnology for Human Empowerment
This year’s theme, “Unlock Your Mind,” spotlights organizations using neurotechnology to enable more independent, empowered lives. Cognixion was selected alongside a group of other innovators advancing ethical, human-centered solutions at the intersection of brain science and real-world impact.
Cognixion’s work focuses on restoring communication and autonomy for people with speech and mobility impairments through wearable brain-computer interface technology—enabling thought-driven interaction, self-expression, and connection.
As part of this recognition:
Cognixion will be featured in a year-long exhibition at The Tech Interactive
Our work will be included in educational programming and materials reaching thousands of students, families, and educators
Visitors will have the opportunity to explore how neurotechnology can expand access, independence, and human potential
2025–26 Tech for Global Good Laureates
The 2025–26 class of laureates includes:
Cognixion (Santa Barbara, CA)
Sanmai Technologies (Sunnyvale, CA)
BCI Pioneers Coalition (Columbus, OH)
Drive Medical / NexStride (Port Washington, NY)
Together, these organizations represent a shared commitment to ethical innovation, accessibility, and technology that serves people first.
A Celebration of Technology for Good
The Tech for Global Good Celebration is The Tech Interactive’s primary fundraiser, supporting hands-on STEM education, scholarships, and inclusive learning initiatives across Silicon Valley and beyond. Proceeds from the event help ensure that future generations can engage with science and technology in ways that are inspiring, accessible, and empowering.
We are deeply grateful to The Tech Interactive for this recognition and excited to share Cognixion’s work with the broader public over the coming year. If you find yourself in San Jose, we invite you to experience the exhibition and see firsthand how neurotechnology can help unlock the mind.